The U.S. Fish and Wildlife Service’s Incident Command Team, in collaboration with partner agencies, continues to develop and implement conservation strategies to help California condors considering Highly Pathogenic Avian Influenza (HPAI).
Incident Update
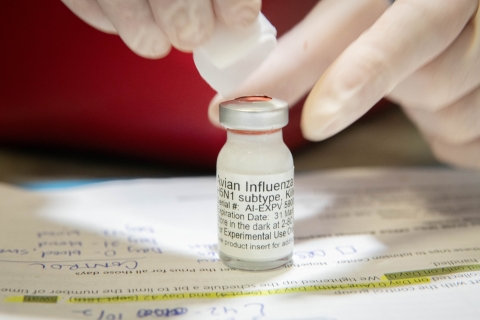
California Condor Vaccination Trial
Final analysis for the samples from Group 1 for condors are complete. USDA conducted hemagglutination inhibition tests on serum samples collected 42 days after the vaccination. The samples were evaluated for antibody titers that are commonly used as surrogate markers against influenza and other pathogens.
Group 1 received a vaccination of 0.5ml on two occasions (initial injection and booster). Results show that 60% of the condors had titers that are expected to provide partial protection against mortality, while 10% of those birds had titers expected to provide protection against mortality.
Group 2 received a single 1 ml dose vaccine. Results pending.
10 condors completed vaccine administration.
10 condors have completed the 42-day trial period.
Group 3 includes control birds. They will not receive vaccines, but blood samples will be collected.
Additional hemagglutination inhibition tests are being conducted to provide a better understanding on the duration the birds may be protected and to what extend from the currently circulating strain of HPAI.
Field Operations
The Service determined that based on the results from Group 1, release birds for this fall and winter will be vaccinated with the Group 1 regimen (two 0.5ml doses) prior to release. The vaccine should provide some level of protection from mortality if the birds are exposed to the virus, and likely decrease the degree to which an individual becomes ill. This will result in 14 additional birds being vaccinated, leading to a total of 26 vaccinated release birds (including those in the trial). Release of individuals will continue to be dependent on the individual’s behavior and conditions at the local release site.
The Service is working with USDA, U.S. Geological Survey, State Veterinarians, and recovery partners to develop site-specific vaccine plans for each of the zoological institutions that care for condors and the release sites so vaccines can be implemented prior to release.
Prior to expanding the use of the vaccine in captive and free-flying condors in future years, the Service will (1) complete the current vaccine trial (2) evaluate changes in the level of antibodies in vaccinated condors over time and (3) evaluate condors for antibody titers in Arizona/Utah population that may have been previously exposed to HPAI and survived.